Development of a new loop-mediated isothermal amplification test for the sensitive, rapid, and economic detection of different genotypes of Classical Swine Fever Virus

Jose Alejandro Bohorquez, Adriana Muñoz, Saraswathi Lanka, Liani Coronado, Rosa Rosell, Mònica Alberch, Carol W Maddox and Llilianne Ganges.
As one of the most important diseases in animal health, Classical Swine Fever (CSF) continues to be a challenge in terms of disease control. It is caused by the CSF virus (CSFV), a ssRNA (+) virus, a member of the Pestivirus genus. Control strategies against CSF are reliant on rapid diagnosis, primarily ELISA testing for anti-CSFV antibodies, as well as molecular detection of viral RNA using real time RT-PCR (RT-qPCR) (WOAH, 2019). While proving very useful, these techniques require the use of specialized equipment that is not readily available in proximity to the farms or slaughterhouses where testing is performed. In addition, the specialized equipment, and highly qualified personnel, to carry out RT-qPCR tests is also not readily available in many developing countries. This greatly increases the time needed to obtain a result, which in turn increases the time to take action to control the infection in the affected herds.
Loop-mediated isothermal amplification (LAMP) PCR poses one of the more interesting alternatives, due to its detection methods. LAMP-PCR can be performed using fluorometric detection, aided by easily portable equipment, or even by colorimetric detection, observable to the naked eye. The aim of this study was to design and validate a new LAMP assay for the fast, sensitive, specific, and economic detection of CSFV RNA in fluorometric, and colorimetric formats to be validated side by side with the CSFV reference RT-qPCR test.
A loop-mediated isothermal amplification (LAMP) PCR technique was developed, with primers designed considering all reported CSFV genotypes. The reaction was tested using both fluorometric and colorimetric detection, in comparison to the gold standard technique. Viral strains from three circulating CSFV genotypes were tested, as well as samples from infected animals. Other pathogens were also tested, to determine the LAMP PCR specificity. Besides laboratory RNA extraction methods, a heating method for RNA release, readily available for adaptation to field conditions was evaluated.
Three primer sets were generated, with one of them showing better performance. This primer set proved capable of maintaining optimal performance at a variety of amplification temperatures (60°C – 68°C). Viral strains from three circulating CSFV genotypes were tested, as well as samples from infected animals (Figure 1). Other pathogens were also tested, to determine the LAMP PCR specificity. Besides laboratory RNA extraction methods, a heating method for RNA release, readily available for adaptation to field conditions was evaluated.
It’s worth highlighting that the LAMP assay, whether by fluorometric or colorimetric detection, showed the similar sensitivity as the CSFV RT-qPCR assay reported by Hoffmann et al., 2005, when evaluating magnetic extracted RNA samples from genotype 1. Conversely, the use of this CSFV-LAMP test in the field using minimal equipment, particularly by colorimetric detection, may be limited when trying to analyze serum samples, since all of these were negative when evaluating boiled serum samples. This may be due to the buffering capabilities of serum, which hinder the variation in the mastermix pH and, therefore, the colorimetric change. Similar issues in colorimetric LAMP assays have been reported in a previous study. This phenomenon can usually be solved by serum dilution, as was the case with the samples from animals infected with CSFV genotype 2 Catalonia strain. Nevertheless, this potential limitation can be overcome by the use of other minimally invasive samples, such as nasal and rectal swabs, that did not show these problems and can be collected in less non-invasive manner and even less training in a farm setting. Additionally, the results using samples from pigs experimentally infected with the CSFV genotype 2 strain evidenced a better performance by the colorimetric detection method rather than the fluorometric (Figure 1). The colorimetric LAMP reaction coincided with 93.3% of the RT-qPCR results and was able to detect viral RNA in all samples, regardless of extraction method, after 14 days of infection. This is particularly encouraging, considering that the viral RNA load in some of the nasal and rectal swabs at 7 dpi was near the limit of detection of RT-qPCR.
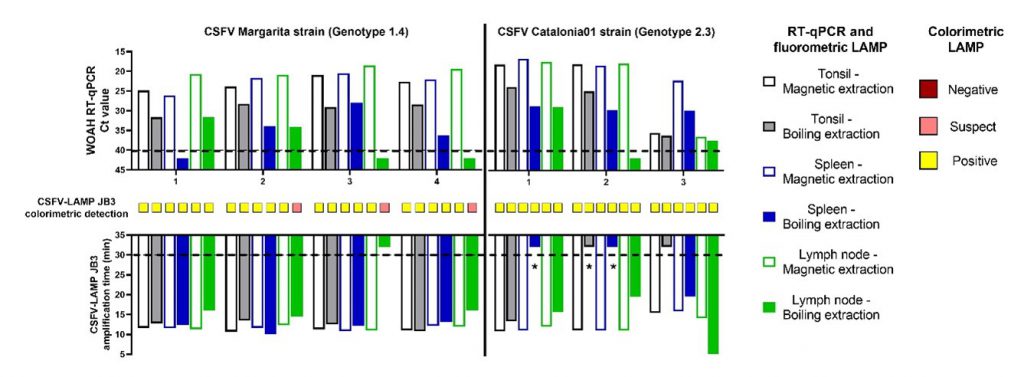
Figure 1. Clinical samples from CSFV experimental in vivo infection.
A potential drawback of the CSFV-LAMP test lies on the unspecific detection of the new OVPV viral RNA. This result is not entirely surprising, given the close genomic proximity of OVPV with CSFV, as well as the fact that RNA from this virus can be detected by the CSFV RT-qPCR previously described by Hoffman et al., 2005. However, with the results obtained with the colorimetric format, is only possible to detect the OVPV in a doubtful result if a large amount of viral load is circulating. It is worth highlighting the rapid capacity of this virus to generate antibodies after infection, which in any case would favor reducing the circulating viral load and therefore, the OVPV cross-reactivity of with test developed here. In addition, it’s unlikely that the cross-reaction of the LAMP test with OVPV would translate to a field setting, since the only study regarding experimental infection of pigs with OVPV showed that the viral RNA load detected in pigs after infection is much lower than that used for testing of the LAMP assay in the present study.
The results from the present study show the development of a novel tool for the detection of CSFV, one that may have important applications in a field setting, where subclinical and chronic forms of disease continue to persist. Some of these forms of disease fail to be diagnosed by the serological techniques routinely used for surveillance programs and their detection relies on nucleic acid amplification. The joined use of novel and traditional diagnostic techniques could prove to be a useful alternative for the early detection of CSFV, lending itself to faster action, having an overall positive impact in the eradication of the disease. Thus, official regulations and guidelines for the use of point-of-care diagnostic tests in surveillance and control programs for notifiable diseases for the WOAH need to be established.
Bohórquez JA, Muñoz-Aguilera A, Lanka S, Coronado L, Rosell R, Alberch M, Maddox CW, Ganges L. Development of a new loop-mediated isothermal amplification test for the sensitive, rapid, and economic detection of different genotypes of Classical swine fever virus. Front Cell Infect Microbiol. 2024 Apr 15;14:1372166. doi: 10.3389/fcimb.2024.1372166. PMID: 38686097; PMCID: PMC11056584.













