24th March, World Tuberculosis Day

Every year the World Health Organization (WHO) celebrates World Tuberculosis Day. The IRTA-CReSA tuberculosis research team explains how daily work is investigating this disease.
Tuberculosis: a known disease in uncertain times (Bernat Pérez de Val)
We are living exceptional days, with good reason we dedicate our time to conjecture on how the COVID-19 pandemic, caused by SARS Coronavirus-2, will affect our lives and, by extension, the whole society. It is a huge challenge that most of the present generations had not even imagined. But we will overcome it, we will learn from all this and what we will have lived will remind us that research into infectious diseases and their control are fundamental in a globalized world.
Not all infectious diseases behave the same, tuberculosis is a clear example, it does not generate large epidemic peaks in a short period of time, it usually progresses slowly in infected people, we could say that it is a discrete, almost silent disease. But it spreads a lot, almost 10 million people get infected every year, it is estimated that a third of humanity on the entire planet is infected, and it also kills, a lot, more than a million and a half people each year, an unbearable number for a disease that should almost always be “curable”.
Tuberculosis is also a disease that people and animals share. It is caused by a group of microorganisms called Mycobacterium tuberculosis complex mycobacteria, and it is included in the zoonotic diseases group, that is, those that can pass from animals to people and vice versa. That is why in Catalonia we have been preventing, controlling and fighting animal tuberculosis for years, starting with bovine tuberculosis, subjected to an eradication program at the state level, continuing with the control of the disease in other production animals (especially goats which are a very important reservoir) and ending with disease surveillance in wildlife, which often interacts with production animals. Below we will discuss some of the activities carried out by the IRTA-CReSA mycobacteria diagnostic laboratory.
How do we grow mycobacteria? (Maite Martín)
The samples that we analyze to be able to isolate the mycobacteria can come from different origins:
- Bovines and goats destined to human consumption, which have tested positive for diagnostic tests.
- Slaughterhouse animals where a lesion has been observed in the animal suspected of tuberculosis.
- Hunted wildlife.
Of all these animals, the preferred sample type that we analyze are lymph nodes, mainly respiratory, to isolate mycobacteria: we homogenize the tissues and culture in selective solid media for the growth of mycobacteria and also in liquid medium, using an automated system called BACTEC MGIT. These methods are the same as those used in hospitals for diagnosing tuberculosis in humans.

Once we obtain growth, we perform molecular techniques (including PCR) to find out the specific strain that each infected animal had. In this way we can make an epidemiological study of outbreaks, for example studying if there are relationships between the isolated strains in the cattle or goat farms and wildlife of a specific area.
The main drawback is that mycobacteria take a long time to grow in culture. In solid medium we cannot give a sample as negative until 3 months have passed since the start of the culture, therefore, another technique we perform is a quantitative PCR directly from the tissue homogenates, which allows us to obtain a preliminary result in 2 days. , although this PCR will not test positive unless there is a relatively high bacterial load. All this activity is carried out in the IRTA-CReSA level 3 Biocontainment Unit, with an FPP3 mask, protective glasses and a suit that even covers our heads, since it is a disease that also affects people.
Looking for mycobacteria under the microscope (Mónica Pérez)
Another method used for the diagnosis of tuberculosis consists of the pathological study through the microscopic evaluation of the tissues. In the event that the pathologist macroscopically observes lesions compatible with tuberculosis, a small portion of tissue will be collected, fixed in formalin and embedded in paraffin.
The histological study consists of these techniques:
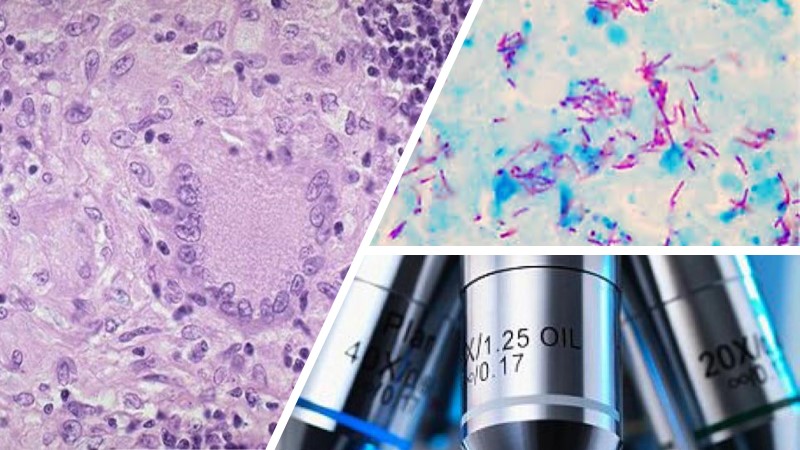
- Microscopic evaluation of the presence of tuberculous lesions by means of Hematoxylin-Eosin (HE) staining that will allow us to make a preliminary diagnosis of tuberculosis. If the result is positive, we proceed to make a differential stain.
- Microscopic evaluation of the presence of acid resistant alcohol bacilli using the Ziehl-Neelsen (ZN) stain. This staining is mainly based on the characteristics of the bacterial wall that mycobacteria have, which give them the property of resisting discoloration with alcohol-acid after staining with a basic dye (phenolic fuchsin). Therefore, “acid resistant alcohol” bacilli (Mycobacteria) will be observed under a microscope in a fuchsia color (Stained with the phenolic Fuchsin) as they will have resisted fading, while other bacteria and cell structures will stain green due to contrast dye (Light Green). The Ziehl-Neelsen stain is a simple, fast and inexpensive technique, although it is of low sensitivity but it is a valuable tool for the pathologist to detect cases that come to the laboratory suspected of tuberculosis.

Can we ‘see’ tuberculosis in the blood? (Zoraida Cervera)
Tuberculosis is a disease that we can detect in the laboratory through various diagnostic techniques, among them are ELISAs, which are immunological techniques that allow us to determine if animals are infected or have been in contact with the Mycobacterium.
In the tuberculosis diagnostic laboratory we use two different ELISAs:
- The ‘interferon gamma’ technique helps us detect an animal with active disease. If so, its immune system will have produced cells that release a cytokine, called interferon gamma, to fight infection. To perform this technique we need animal blood that we have to process in the laboratory within 8 hours of its extraction and then we stimulate this blood with some proteins from the mycobacterium, if the animal was infected, gamma interferon will be released into the blood that we will detect the following day by ELISA technique.
- The other technique we use is the tuberculosis antibody detection With this technique we also detect very chronic animals that can no longer be detected by the gamma interferon technique. To perform this technique we need the blood serum, since it is where we can detect the antibodies that the animal will have generated to fight the infection.
Looking for mycobacteria in wildlife (Abel Muñoz)
One of the procedures that are of vital importance for tuberculosis control is the analysis of samples from wildlife. This will allow us to control the different points of infection of the mycobacterium in the natural environment. We obtain the samples thanks to the help of hunters and rural agents, mainly, since they send us the lymph nodes and the blood of their captures.
When we receive the samples, the first thing is to organize them, the blood will be used for antibody detection techniques, while the tissues will be analyzed to verify that they do not present macroscopic lesions compatible with tuberculosis. The procedure followed with the blood tubes begins with separating the serum, which contains the antibodies that the infected animals have generated against the mycobacterium and which is what we want to detect to find out if the animal had been in contact with the disease.
With the tissues, the procedure begins by making a detailed exploration through different serial cuts only a few millimetres thick, this will allow us to closely observe the interior of the lymph nodes, avoiding the neglect of any small lesion. In the case that we find any lesion compatible with tuberculosis, we will proceed to take a sample in order to check if by cultivating it we can obtain the growth of the tuberculous mycobacterium and give the sample as positive for tuberculosis. Also histopathological evaluation will be conducted to confirm the presence of compatible microscopical lesions, this allows us to ruel out TB by detecting other causes.
Once the procedures are finished, it is important to record the data obtained in order to plan the measures that will be carried out to monitor and, as far as possible, eradicate tuberculosis in natural settings where positive cases have been evidenced, and also to study the interaction that exists between different positive animal species from the same areas.
The fight goes on
With this work in the laboratory and much more that we have not explained, at IRTA-CReSA we fight against this ancient disease that today continues to be a great challenge in animal health and public health. The fight goes on, we will continue to fight it.













