Animal models of post-COVID-19 condition
Now that the emergency phase of the COVID-19 pandemic has officially ended, we are left to face its long-term consequences.
About 40% of patients who had COVID-19 in the past three years have experienced symptoms months after the infection. This condition was initially defined as long-COVID by the patients – the first to raise attention to their long-lasting symptoms using social media – and is now known as post-COVID-19 condition (PCC).
The most common symptoms of long-COVID are fatigue, shortness of breath, pain, increased heart rate, altered sense of taste and smell, headache, and a long list of neurological and psychological impairments. Patients often describe their difficulty with concentration and short-term memory using the powerful expression “brain fog”; sleep disorders, anxiety, and depression are also common complaints.
These symptoms can either persist after the acute infection (with negative COVID-19 tests at this point) or develop weeks after the end of the disease, even if it was mild. Given the hundreds of thousands of individuals who have been infected by SARS-CoV-2 so far, the number of people at risk of suffering long-term consequences is very high. PCC represents, therefore, not only a healthcare burden but also a societal problem, since it severely impacts the quality of life, reducing the ability to work and engage in social activities. Interestingly, PCC is more frequent in females, while the chances of having severe acute COVID-19 are higher in males.
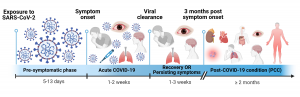
Fig. 1 | Timeline of acute COVID-19 and PCC in humans.
The causes and mechanisms of PCC are yet to be characterised at the molecular level, leaving patients suffering from this condition without proper treatment. The study of animal models, extremely helpful in understanding acute COVID-19 and in testing vaccines and treatments, is helping to unveil some of the aspects of PCC. To date, no animal model fully reproduces PCC as it occurs in humans, but several studies conducted on different species have yielded interesting results.
Our group has recently published an article on Lab Animal, summarising the current knowledge on this topic, focusing on the neurological manifestations of PCC, and discussing the applicability of the existing animal models to further understand its molecular and cellular mechanisms.
For example, hamsters have been extensively used as models for COVID-19. It has been found that hamsters infected with SARS-CoV-2 had a modified expression of specific genes in the brain one month after infection when the virus was no longer detectable in the organism. The genes more expressed in these animals than in hamsters that were never infected by SARS-CoV-2 are normally activated during inflammation. Another study has found inflammation and accumulation of altered forms of two proteins called tau and alpha-synuclein in the brain of hamsters two weeks after infection with SARS-CoV-2. These results are particularly interesting because the accumulation of modified tau and alpha-synuclein are characteristic findings in the brain of patients with neurodegenerative diseases (like Parkinson’s and Alzheimer’s), suggesting similar mechanisms between PCC and these conditions.
Moreover, behavioural studies have shown that infected hamsters have reduced spontaneous activities and increased sensitivity to pain, resembling some of the neurological symptoms reported by patients, probably due to a continuous inflammation of the nervous system.
Similar studies in mice have found long-term inflammation in the brain after infection with SARS-CoV-2, even when the respiratory disease was mild, and the virus was not detectable in the brain. Once again inflammation was accompanied by altered gene expression suggestive of cognitive impairment like in aging and Alzheimer’s disease.
Importantly, most of the studies published so far involve non-vaccinated animals infected with the original strain of SARS-CoV-2, but since the beginning of the pandemic several variants have emerged, and a growing number of people have been vaccinated worldwide, improving our ability to fight the infection.
This brings up two important questions:
- Do the differences between variants influence the probability of developing PCC and the intensity of the symptoms?
- Since PCC can still occur when infected after vaccination, is it substantially different from the disease developed by non-vaccinated individuals?
To really understand how to tackle the long-COVID problem, more than one animal model will be necessary, with different combinations of variants, vaccination status, and genetic backgrounds.
It is not an easy task: with so many factors to consider and such a wide range of possible outcomes, complex experiments will be needed.
Together with the other members of the EU-funded EPIVINF project, we are working to understand a specific aspect of PCC: the impact of SARS-CoV-2 on the regulation of the host gene expression and how it can affect neurological health in the long term.
Our article: Animal models to study the neurological manifestations of the post-COVID-19 condition | Lab Animal (nature.com)
EPIVINF website: EPIVINF – Understanding the epigenetic regulation to fight viral infections
Cover image: Imagen de pch.vector en Freepik













