New edition of the Biosafety and Biosecurity Module of the IDOH master’s degree at IRTA-CReSA

Biosafety and bioprotection are issues that have gained a space for the attention of the general public as a result of epidemics that have spilled from their usual compartments, pandemics and other global threats to health, all this seasoned with the permanent sword of Damocles of a malicious use of knowledge to improve or disperse lethal pathogens for bioterrorist or criminal purposes.
There are very few initiatives that address Biosecurity and Bioprotection in the university environment and even less those that allow attendees basic work in a high biological containment unit. And this is what has taken place in the last two weeks (between May 27 and June 7) at the facilities of IRTA-CReSA, within the practical module of Biosafety & Biosecurity in the Master’s in Infectious Disease One Health (IDOH) Erasmus Mundus. One of the four semesters of the master’s degree takes place at the Autonomous University of Barcelona and one of the modules, which is addressed to us, is led by IRTA-CReSA staff with support from a renowned expert on risk assessment at Jurgen Mertsching, of the Faculty of Medicine of Hanover.

The module consisted of master classes in the morning, and practical activities within the Biocontainment Unit. In the morning all the lessons articulated around two main axes: the biocontainment and biosecurity associated to the facilities on the one hand and the evaluation of risk as a key technique in the management of the biological risk on the other.
In terms of biocontainment and biosecurity, the categorization of microorganisms was discussed based on their effect on humans and animals; the description of the levels of Biosecurity or containment and the relation between these and the previous categorization; how to safely do the removal and closure of high-security facilities; inactivation processes; management of solid, liquid and gaseous waste; transportation of infectious biological material and its categories; Individual protection equipment (EPIs) and finally the particularities of high-contention facilities that work with large animals (for example, calves, pigs, goats, sheep, alpacas, etc.) as opposed to small animals of laboratory (rat, mouse, rabbit, etc.).

But a crucial element, once “having” an installation is the management of biological risk and this can only be done effectively with a rigorous but flexible application of the tool that is the risk assessment. Thus, we talked about the differences between Biosecurity and bioprotection, which is exactly a risk assessment, risk assessments applied to diagnostic and hospital labs, performance gain experiments as a clear example of the controversy between risk assessments Several, practical exercises of group risk assessments on already published Open Access articles, and finally a description with associated discussion of the quasi-definitive draft of the 4th edition of the Laboratory Biosafety Manual (LBM) of the World Health Organization, which seems to be about to be published.
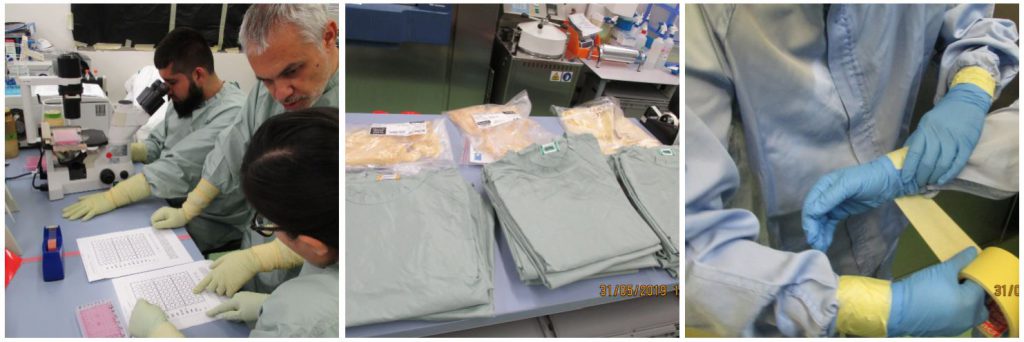
However, possibly the differential element of this master’s degree is that it allows students to “work” under continuous supervision within a high biological containment facility. Under the tutorial, they learn the processes of entry and exit of the installation, how to correctly set EPIs, the basic work in the biological safety cabin and the progressive improvement of its dexterity, the propagation of virus in cell culture, detergent inactivation processes, nucleic acid lysis tampons, or thermal ones, and how to quantify them by cell culture, decontamination of CSB, or how to deal with biological incidents (theatrical simulation) among others.

A total of 22 students from all over the world (Germany, Australia, Bangladesh, Belgium, Botswana, Brazil, Cameroon, Egypt, Philippines, Great Britain, Iraq, Mexico, Nepal, Nigeria, Pakistan, Portugal, USA …) have received this training will allow them to face Biosafety and Biocontención with new knowledge and tools. They will never be able to eliminate everything from the risk, but we can value it in all its facets, because the “zero” risk obviously does not exist.
But this one, this is another story.

Practical case and monitoring of biological spillage (simulation), through fluorescence solution.













