Breakthroughs in the development of the BA71ΔCD2 vaccine against African swine fever, as well as in the knowledge of the immunity associated with protection

African swine fever (ASF) is a lethal viral disease of mandatory notification to the World Organization for Animal Health (WOAH, former OIE), currently causing a pandemic with devastating consequences for the swine industry worldwide. The disease is caused by the ASF virus (ASFV), affecting pigs and wild boars with a mortality rate close to 100% in its acute forms. There is currently no effective vaccine approved globally. Therefore, control of the disease relies on the implementation of drastic biosecurity measures and sanitary depopulation policies in affected areas.
The group led by Dr. Fernando Rodríguez (IRTA-CReSA), alongside with Dr. Jordi Argilaguet, several scientific collaborators, and the international company Huvepharma, has developed a vaccine prototype based on a live attenuated virus in which the CD2v protein of the parental virulent virus BA71 (BA71ΔCD2) has been eliminated. This recombinant virus differs from others generated by other laboratories in its ability to not only protect pigs against infection with the homologous virulent strain (BA71), but also to provide cross-protection against lethal infection with strains of other genotypes, including the virulent pandemic virus (Georgia2007). The first innovative feature of our work is that intranasal inoculation of BA71ΔCD2 has proven to be much safer than intramuscular inoculation (most used route of administration), maintaining its high efficacy, even against lethal infection by contact with pigs infected with pandemic ASFV. So far, these live vaccines based on recombinant viruses attenuated by deletion of viral genes and inoculated intramuscularly, are the only immunization strategy that has demonstrated high efficacy. In fact, one of these vaccines (Navet-ASFVac) has recently been licensed for its use in Vietnam. However, the use of this vaccine was suspended due to the occurrence of ASF outbreaks possibly associated with flaws in the vaccination protocols followed on some farms, thus confirming the main limitation of this vaccine strategy: the biosafety concerns arising from its very live nature. Therefore, it is of vital importance to continue researching with the aim of diminishing the biosafety problems associated with live attenuated vaccines. Our study incorporates a breakthrough in this regard: simply by changing the route of administration from intramuscular to intranasal, the safety of the vaccine is exponentially improved, at least in the case of BA71ΔCD2. Simultaneously to the development of these type of vaccines, which will undoubtedly be able to help control the disease in affected or at-risk areas in the short to medium term, we continue to make progress on the vaccines of the future based on subunits. These vaccines are much safer, but require much more investment in R+D+i, to both characterize the immunogenic viral antigens to be used as well as to improve the knowledge of the immunological mechanisms associated with protection against ASFV.
The second objective of the study was to characterize the cellular immunological mechanisms associated with protection induced by intranasal vaccination with BA71ΔCD2. So far, several studies had shown that CD8+ cells play a role in protection. However, the main immunological components and functional mechanisms associated with this protection remained unknown. Thanks to a close collaboration with Dr. Anna Esteve-Codina’s bioinformatics group (CRG-CNAG), in this study we performed transcriptomic studies both at the population level (bulk RNAseq of blood cells) and individual cells (single cell RNAseq of submandibular lymph node cells) that allowed us to characterize the immune response induced by the vaccine. For this purpose, cells from vaccinated and unvaccinated pigs were stimulated in vitro with ASFV in order to characterize the vaccine-specific response induced by a second encounter with the virus. The results showed that the vaccine induces two cell types that are activated after specific stimulation with the virus: on the one hand, polyfunctional Th1 memory cells (CD4+CD8+) that produce the cytokines IFNG and TNF; and, on the other hand, cytotoxic cells with the capacity to kill infected cells. Finally, we also observed that, along with this adaptive immune memory response, a macrophage-mediated inflammatory response is activated. Furthermore, we were able to demonstrate that macrophage activation is dependent on IFNG-mediated signaling, indicating that the observed inflammatory response is dependent on the prior activation of IFNG-secreting vaccine-induced Th1 cells. Although the relative role in protection of each of these immunological components has not yet been evaluated, these results represent an important advance in the understanding of ASF immunity.
Overall, this study represents a breakthrough in two important aspects of ASF research. On the one hand, it demonstrates that intranasal vaccination with live attenuated BA71ΔCD2 virus is safe, immunogenic, and effective against a lethal challenge similar to those occurring in affected farms. While efficacy and safety studies required for registration are ongoing, it should be recalled that it is the only experimental vaccine that has demonstrated efficacy against the two genotypes currently circulating in Asia and Europe. On the other hand, the detailed characterization of the immune response associated with protection opens the door to further studies focused on identifying the immunological components that correlate with protection, a crucial unresolved issue. This knowledge would not only allow the evaluation of vaccine candidates without the need to infect animals but is also of great importance for the rational development of future vaccines.
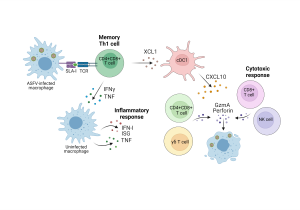
Graphic Abstract – Cross-protection against African swine fever virus upon intranasal vaccination is associated with an adaptive-innate immune crosstalk
Cross-protection against African swine fever virus upon intranasal vaccination is associated with an adaptive-innate immune crosstalk
Laia Bosch-Camós, Uxía Alonso, Anna Esteve-Codina, Chia-Yu Chang, Beatriz Martín-Mur,Francesc Accensi, Marta Muñoz,María J. Navas, Marc Dabad, Enric Vidal, Sonia Pina-Pedrero, Patricia Pleguezuelos, Ginevra Caratù, María L. Salas, Lihong Liu, Stanimira Bataklieva, Boris Gavrilov, Fernando Rodríguez, Jordi Argilaguet
Published: November 9, 2022
https://doi.org/10.1371/journal.ppat.1010931
Post authors: Uxía Alonso, Laia Bosch, Fernando Rodríguez i Jordi Argilaguet













